VIDEO: Read and understand the nuclide map
What is in a nuclide map?
A nuclide map provides information on the Nuclear physics extremely important nuclides. All atomic nuclei are called nuclides. The following criteria are used to distinguish between nuclides.
- Ordinal number Z: This indicates the number of all protons in a nuclide. Another term with the same meaning is the atomic number.
- Neutron number N: This means all neutrons in a nuclide.
- Mass number A: It indicates the number of all nucleons. D. H. of all protons and neutrons together.
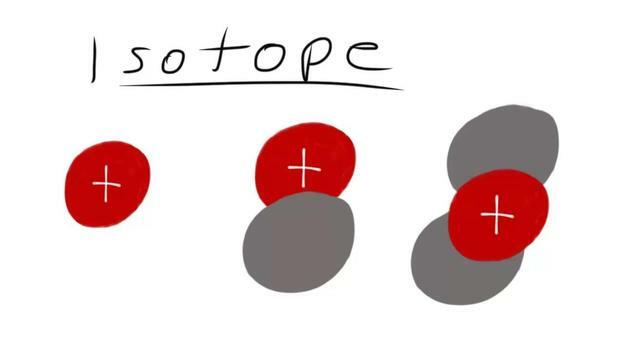
- Nuclides with the same atomic number but different numbers of neutrons are called isotopes.
- With the same mass number but different atomic number, they are isobars. These have roughly the same mass.
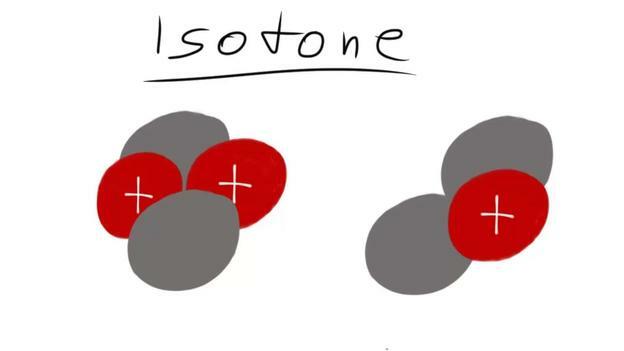
- If, on the other hand, the number of neutrons is the same, but not the number of protons, one speaks of isotons.
- There are stable and unstable nuclides. The unstable ones are called radionuclides because of their radioactivity and decay with emission of α, β and γ radiation until the decay series ends in a stable state.
What is the difference between the isotopes U235 and U238?
Can you really differentiate between the two uranium isotopes U235 and U238 and what is ...
How to read information from the card
- All isotopes (same atomic number / number of protons) are listed horizontally in ascending order on the x-axis.
- All isotons (same number of neutrons) are shown on top of each other, ascending vertically in the direction of the y-axis.
- Similarly, isobars are found on a descending straight line (same mass number / number of nucleons).
- In a box for a nuclide you can find the element symbol and the number of nucleons at the top. Including the frequency of the isotope in percent for a stable nuclide and the half-life for unstable nuclides. Below are the energies depending on the type of decay in megaelectron volts.
- The individual background colors stand for different decays, which are usually also indicated (black often stands for stable nuclides). Otherwise one can assume stable nuclides that have no half-life and the others Colors by reversing the determination of a decay series described in the following assign.

Determine a decay series using the nuclide map
In the case of a decay series, you read out which type of decay is present and can then orientate yourself on the coordinate axes of the nuclide map in order to determine the decay products. Repeat this process until a stable nuclide is obtained or no further products can be determined.
- α-decay: Here the nuclide loses two protons and two neutrons. (-2p, -2n)
- β-minus decay: a neutron becomes a proton. (+ 1p, -1n)
- β-plus decay: a proton becomes a neutron. (-1p, + 1n)